Science
Related: About this forumA Nice Chemical Engineering Paper on the Hydrogenation of Carbon Dioxide to Methanol.
In a famous paper, the Nobel Laureate George Olah, one of "The Martians,", proposed a closed carbon cycle based on a methanol/dimethyl ether (DME) economy.
Anthropogenic Chemical Carbon Cycle for a Sustainable Future George A. Olah, G. K. Surya Prakash, and Alain Goeppert Journal of the American Chemical Society 2011 133 (33), 12881-12898.
This paper has been cited well over 1000 times.
Before proceeding too much further with this post, I note that Olah's advocacy of a closed carbon cycle involves something that I often criticize in this space, the idea is built around storing energy and transporting energy, and not about primary energy. In this, it is similar to the ridiculous and much hyped nonsense about batteries and even worse, consumer hydrogen, both of which are very, very, very popular and very, very, very stupid, particularly because they make the use of dangerous fossil fuels, which remains the dominant source of primary energy, worse, not better by virtue of the 2nd law of thermodynamics.
There is one, and only one, sustainable form of primary energy, nuclear energy.
One of nuclear energy's disadvantages is also one of its strengths, high temperatures of the fuel. As a strength, which can be addressed with advances in Materials Science, using these high temperatures hydrogen for captive use can be captured by raising the thermodynamic efficiency of the system using heat networks, in which case, as I have also often argued here, exergy destruction is minimized under conditions by which electricity becomes a side product, rather than the main product of nuclear energy. One major product would be hydrogen for captive use. Hydrogen has no properties to recommend it as a consumer product, but many to recommend it as a captive synthetic intermediate, which overwhelmingly is and remains and should remain its only use.
This brings me to the paper I wish to which I wish to refer, this nice chemical engineering paper that refers to the best type of process chemistry from an economic and environmental sense, continuous flow chemistry:
Modeling and Simulation Based Parametric Analysis for Monolithic CO2 Hydrogenation Reactors Using Experimental Data Usman Salahuddin, Ralph Shortt, Chunxiang Zhu, Dongsheng Li, and Pu-Xian Gao Industrial & Engineering Chemistry Research 2023 62 (36), 14300-14310
From the paper's introduction:
Although different types of catalysts (4,5) and reactors have been reported in the literature for CO2 hydrogenation, such as slurry reactors, (6) fluidized bed reactors (7) and membrane reactors, (8) Cu/ZnO/Al2O3 pellet-based catalysts are the most widely used commercial catalyst in a packed bed reactor for direct CO2 hydrogenation into methanol. (9) Typical reactors have three types of resistances, namely, kinetic resistance, external mass transfer resistance, and internal mass transfer resistance. (10?12) Nevertheless, overcoming internal and external mass transfer resistances has always been a challenge in packed bed reactors. Gas channeling, boundary layer formation, and internal diffusion are a few of the many factors that affect reactor performance and are driven by these resistances. (13) To improve the reactor performance, an effective reactor design is needed that minimizes these resistances to help maximize the reactant conversion, product yield, and selectivity.
Monolith catalytic reactors have been widely used in various applications such as vehicular and stationary engine exhaust aftertreatment, chemical and fuel processing, and catalytic oxidation of volatile organic compounds. (14) Monolithic reactors offer uniform catalyst coating and efficient material usage and can accommodate lower particle size without incurring high pressure drops, unlike pellet-based packed bed reactors. Fan and Tahir (15) and Liang et al. (16) have discussed the benefits of using monolithic catalysts and reactors. Du et al. (17) have specifically showed the use of a ZnO nanorod array grown on cordierite monoliths as a support for Cu–ZnO–Al2O3-based CO2 hydrogenation catalyst. Meanwhile, Durán Martínez et al. (18) as well as Lu et al. (12) have used modeling and simulation-based approaches to show the mass transfer benefits of monolithic reactors. As of late, effective use of monolithic reactors has recently drawn attention for CO2 hydrogenation processes, (16,17) but in-depth studies are rather limited pertaining to mass transfer resistances on the process and reaction kinetics, to say the least.
Herein, a three-dimensional (3D) monolithic reactor model was demonstrated for direct CO2 hydrogenation. Parametric fitting models were successfully developed to fit experimental data to the kinetic models...
The de rigueur reference to so called "renewable energy" is noted; this genuflection to popular religion being requisite in grant applications, but nonetheless, the paper is useful.
The reactions for the hydrogenation of carbon dioxide to methanol are given as they often are in this papers:
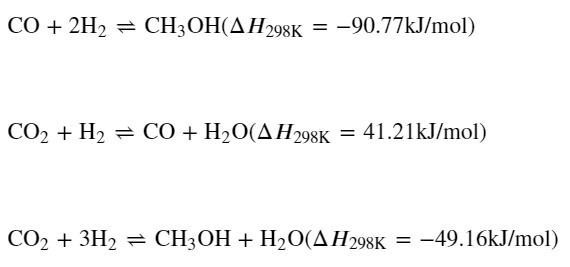
It is noted that two of the reactions are exothermic, and one endothermic, with the heat from two exothermic reactions driving the endothermic reaction. I recall, but will not take the time to cite, a paper by Olah stating that in the case of the similar reactions to give DME directly, the endothermic reaction is mechanistically required, the reaction mixture must contain CO2 in at least trace amounts.
Some graphics from the paper:
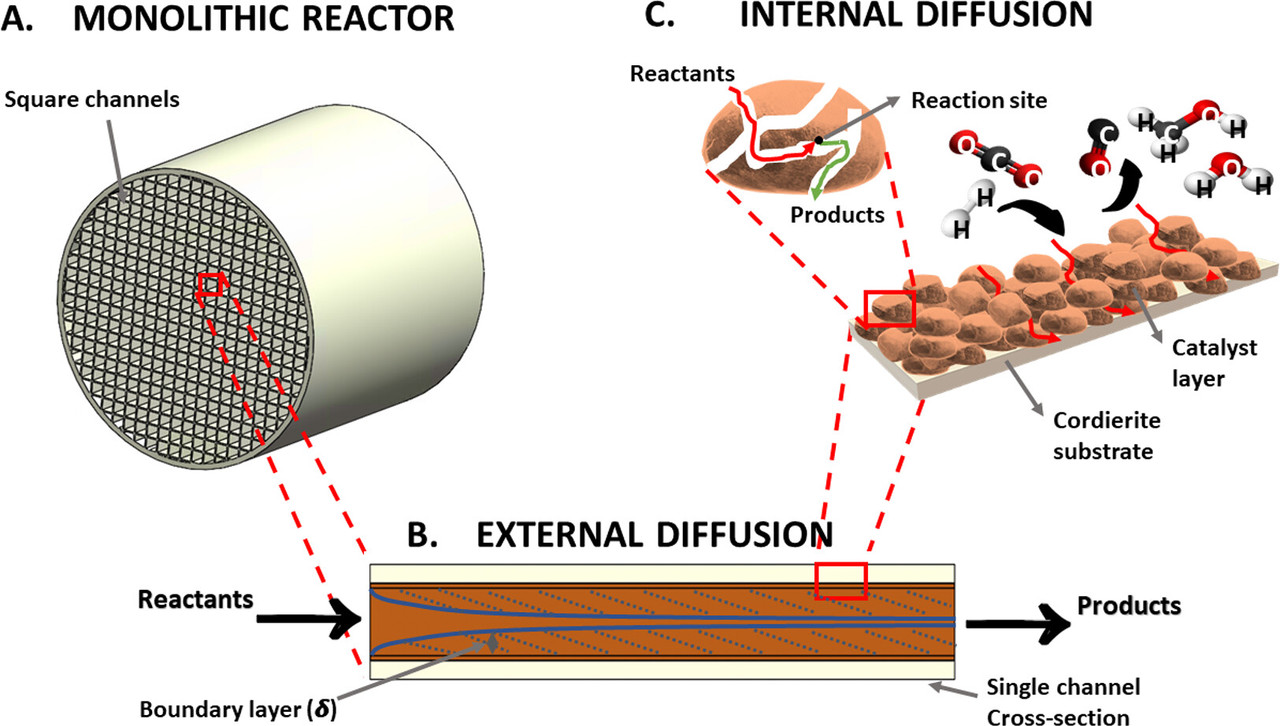
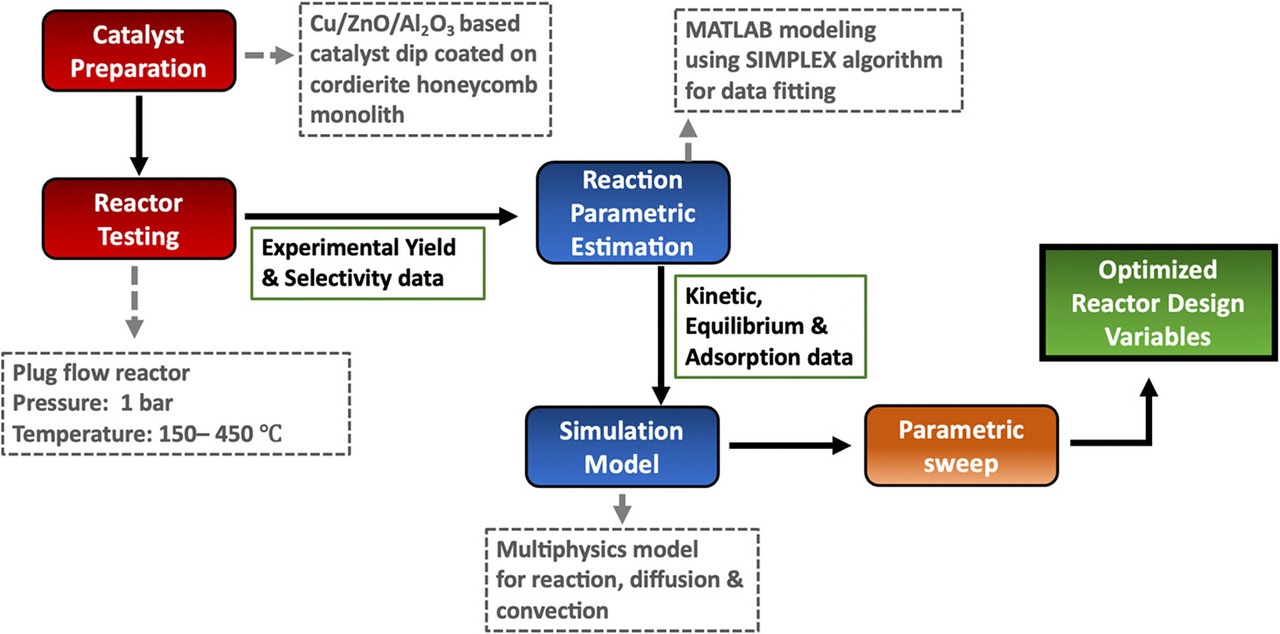
The reaction is reportedly optimized at 300°C.
Some other parameters the paper were examined, this one a very cool analysis of channel geometry:
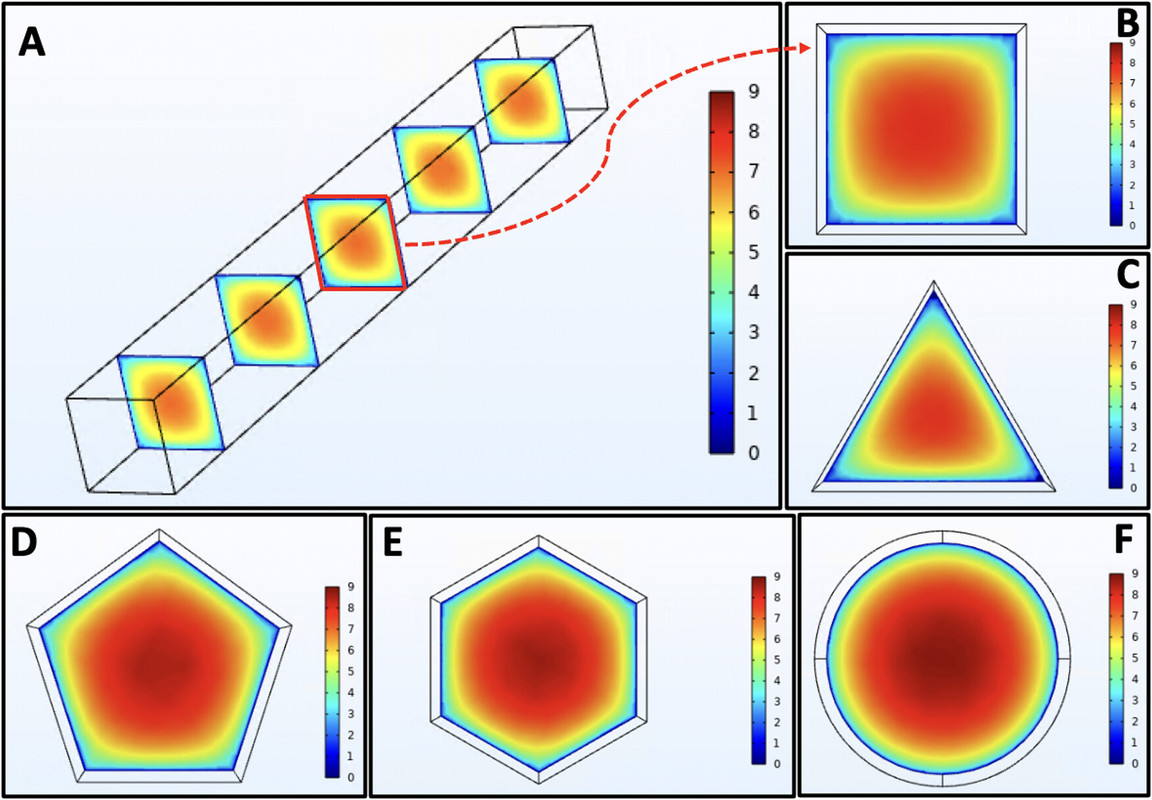
Interestingly and happily, high flow rates give the best yields.
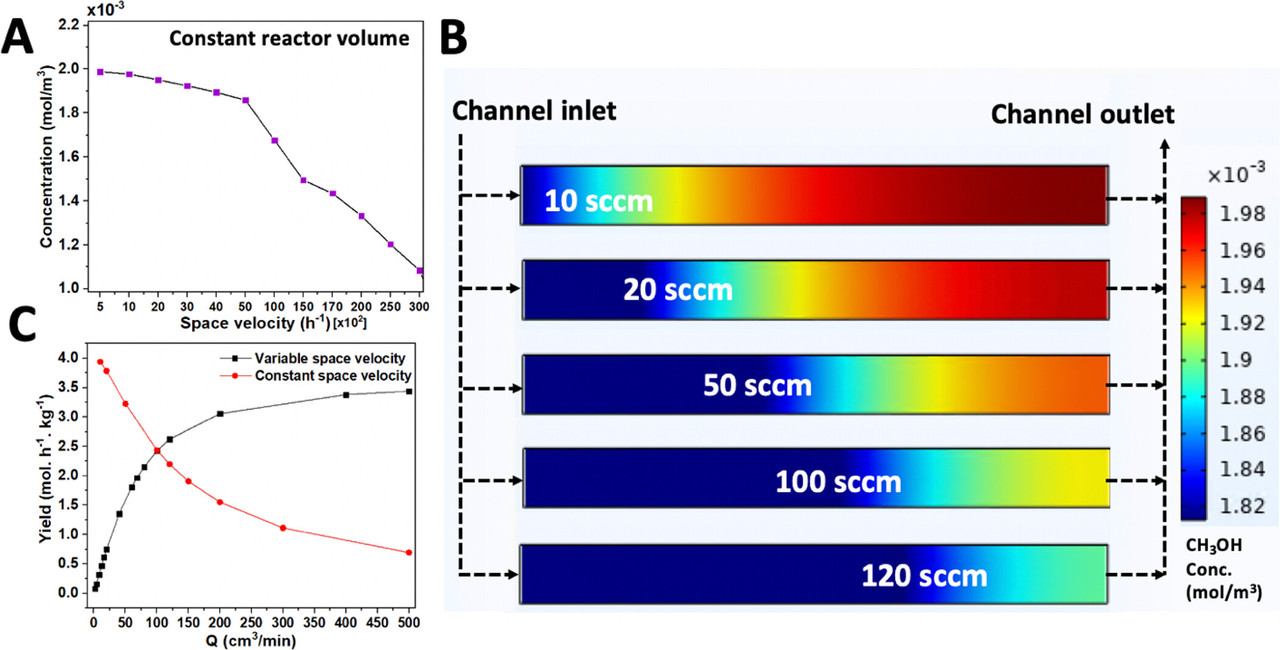
Cool paper, I think.
Have a nice Sunday.
BlueIn_W_Pa
(842 posts)Thank you, NNadir
Warpy
(113,130 posts)I posted an article a week or so ago about the Maersk company converting its small to medium container ships to methanol fuel. Perhaps they were thinking of this process when they launched their first methanol fueled ship and plans to convert the rest of the fleet.
That's definitely a cool paper. Thanks.
NNadir
(34,676 posts)Methanol is probably mostly manufactured by the partial oxidation of methane and, where the hydrogenation of carbon dioxide or carbon monoxide is practiced, the hydrogen is almost certainly made from the reformation of dangerous fossil fuels.
Over in the Ennui and Excuses forum energy storage is more popular than clean primary energy, and I translate that into a de facto endorsement of fossil fuels.
In fact, fossil fuel sales people and sales bots have free reign there.
eppur_se_muova
(37,436 posts)The 3rd eqn you show is the sum of the first two.
Shouldn't there be two steps for
CO + H2 --> H2CO
H2CO + H2 --> CH3OH
I believe those would both be exothermic, and sum to give the first eqn you show. Start with your 2nd eqn, then these two; add all three to give your third eqn.
NNadir
(34,676 posts)I don't have the reference handy, but could get it. I think it referred to MeOH, although it could have been DME. Olah showed that CO2 needed to be present at least in minute amounts, for catalysts to activate.
The direct use of CO2 of course results in the formation of more water which introduces a separation penalty, but happily, the MeOH/water system is zeotropic, unlike the azeotropic EtOH/water system.
The ease of separation from water and the vastly reduced toxicity of DME - dimethyl ether - for which MeOH is a precursor, makes DME superior, as does the easy transformation back and forth from the gas and liquid phases.
There are catalysts for the direct preparation of DME from syn gas, but heat removal has been a challenge. One can drive a turbine with this reduction, a sort of "anti-combustion."