Science
Related: About this forumWunderbar! A Hydrogen Based Perpetual Motion Machine.
I came across this paper in the primary scientific literature: High-Stability ZnAl2O4 Spinel-Supported Nickel Catalyst for High-Temperature Syngas Methanation Gengrui Zhang, Yan Li, Yong Chen, Xinning Hao, Xianhua Zhang, Shuai Wang, Jingdong Lin, Yong Wang, and Shaolong Wan Industrial & Engineering Chemistry Research 2023 62 (41), 16668-16675.
We hear, regrettably, about "green hydrogen," which more or less doesn't exist on any scale that matters. The hydrogen salespeople and salesbots who come to DU to sell fossil fuels by rebranding them as "hydrogen" are selling something worse than snake oil, something worse than "health cigarettes."
A Giant Climate Lie: When they're selling hydrogen, what they're really selling is fossil fuels.
Hydrogen is not primary energy and as such its manufacture destroys exergy meaning that one uses more fossil fuels to make the intermediate hydrogen than one would have used to have combusted the dangerous fossil fuels directly. Almost all of the hydrogen on this planet is made by steam reforming (at high temperatures) of fossil fuels:
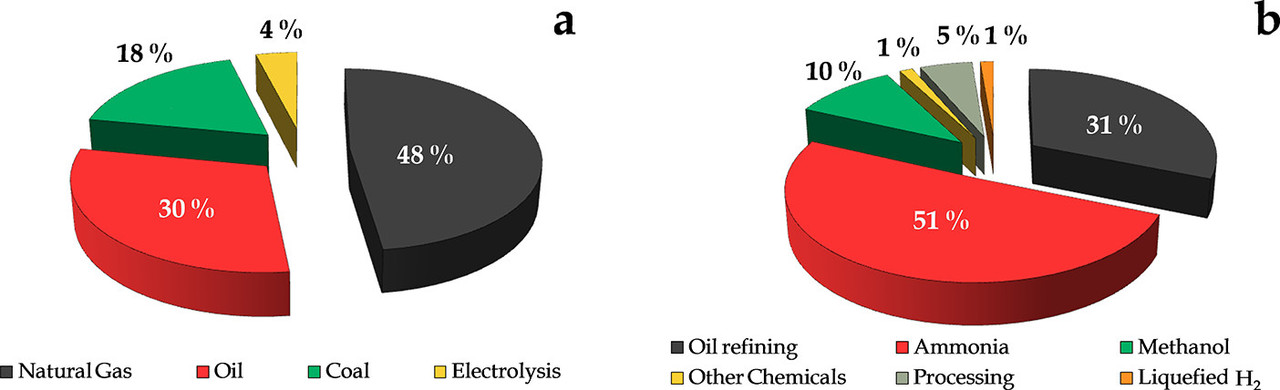
The caption:
Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review Laura Santamaria, Gartzen Lopez, Enara Fernandez, Maria Cortazar, Aitor Arregi, Martin Olazar, and Javier Bilbao, Energy & Fuels 2021 35 (21), 17051-17084]
And now we have this paper that tells us we can make synthetic dangerous fossil fuels, in this case methane, from, um, hydrogen.
To meet this formidable challenge, supported catalysts using varied metals such as Rh, Ru, Ni, Co, etc. have been intensively explored, (6?11) and so are the effects of varied supports including Al2O3, ZrO2, TiO2, SiO2, etc. (12?18) Among the large number of catalysts investigated for the reaction, nickel-based catalysts have been recognized as the most appropriate ones for methanation due to their high catalytic activity, high selectivity to methane, and relatively low price. However, it is known that conventional nickel-based catalysts can deactivate rapidly because of the sintering of Ni particles (during the reduction pretreatment and/or reaction) and carbon deposition. To improve the activity and stability of the nickel/alumina systems, lots of studies have been conducted to optimize the composition, the preparation method, and the catalyst support, (19?23) as well as the addition of promoters such as Ce, Zr, La, etc. (24?29) However, there are still limited reports on the complete syngas methanation over Ni-based catalysts at temperatures above 450 °C, probably due to the formidable challenge confronted in this process. Gao et al. investigated the effect of the structures and surface properties of Al2O3 supports calcined at different temperatures and found that the Ni catalysts supported on ?-type Al2O3 obtained by calcining the commercial ?-Al2O3 at 1200 °C are most active and thermally stable in CO methanation. (30) However, the surface area of the obtained support is very small (only 9.4 m2/g), and notable aggregation of Ni particles occurred after the hydrothermal treatment (particle size increased from 17 to 31 nm), which led to a partial loss in activity. Liu et al. investigated the Ni–Mg/Al2O3 catalysts prepared with different methods and revealed that dispersed NiO interacting with MgO and the support would prevent the agglomeration of Ni crystallites and was likely to improve the activity and stability of the catalyst at high temperatures (up to 700 °C) for methanation. (23) Nonetheless, the major species of the support that reacts with dispersed NiO remained unclear.
The scarce successful examples in the literature thus reveal a need to develop a useful method to prepare highly efficient nickel catalysts for high-temperature syngas methanation. In previous work, we synthesized supported PdZn catalysts using a ZnAl2O4 spinel as a support to provide atomic-level control of the zinc source for the formation of PdZn alloy, thanks to the unique spinel structure and the strong interaction between the metal and the support. The advanced Pd/ZnAl2O4 catalysts show superior activity and stability toward Syngas conversion and methanol-steam-reforming reaction, even upon ppm Pd loading. (31,32) Herein, we came up with a novel design to synthesize a ZnAl2O4-supported/dispersed Ni metal catalyst, where the zinc aluminate spinel featuring superior thermal and hydrothermal stability is employed to support and disperse the active Ni component. The obtained Ni/ZnAl2O4 demonstrated comparable reactivity but better stability during the methanation test conducted above 450 °C, compared with the Ni/Al2O3 counterparts. Furthermore, after high-temperature steam treatment, 35% Ni/ZnAl2O4 still maintains high activity, while 35% Ni/Al2O3 no longer has activity. The strong interaction between Ni and ZnAl2O4 can not only suppress the sintering of Ni particles but, more importantly, prevent the formation of significant NiAl2O4 that causes permanent activity loss during the methanation process...
Actually, I'm fairly sure the authors of this paper are well aware that perpetual motion machines don't work. This is a scheme to make dangerous natural gas from coal, the overwhelming, by far, source of hydrogen in China. The environmental costs of this scheme are, of course, appalling, but don't kid yourself, it could go industrial.
This scheme is every bit as bad as the idea of using hydrogen as a consumer fuel. It is true however, that methane's physical properties, although appalling, are superior to those of hydrogen itself, as the critical temperature of methane is 190 Kelvin, whereas that of hydrogen is 33 Kelvin.
We live in a time where scientific illiteracy is so high that people actually believe that anything, including perpetual motion machines, can be excused by reference to hydrogen.
Hydrogen is an exceedingly dirty fuel since making it wastes primary energy, most of which on this planet, is still generated using dangerous fossil fuels.
Have a nice Saturday evening.
brush
(57,495 posts)It's possible there are hydrogen deposits all over the earth, just as oil deposits arel
https://www.google.com/search?q=recent+pure+hydrogen+deposits+found&client=firefox-b-1-d&sca_esv=581468142&ei=WURPZcLoNa2rur8P4MiCsAI&ved=0ahUKEwiC-dGlzLuCAxWtle4BHWCkACYQ4dUDCBA&uact=5&oq=recent+pure+hydrogen+deposits+found&gs_lp=Egxnd3Mtd2l6LXNlcnAiI3JlY2VudCBwdXJlIGh5ZHJvZ2VuIGRlcG9zaXRzIGZvdW5kMgUQIRirAjIFECEYqwJI6_sBUIsfWPXxAXAGeACQAQCYAX2gAYMbqgEEMzEuN7gBA8gBAPgBAcICCxAAGIoFGIYDGLADwgIIEAAYigUYkQLCAggQLhiKBRiRAsICBxAuGIoFGEPCAgsQLhiABBixAxiDAcICCxAAGIAEGLEDGIMBwgIIEC4YgAQYsQPCAhEQLhiABBixAxiDARjHARjRA8ICBRAAGIAEwgILEC4YgAQYxwEY0QPCAgcQABiKBRhDwgIIEAAYgAQYsQPCAg4QABiABBixAxiDARjJA8ICCBAAGIoFGJIDwgILEAAYigUYsQMYkQLCAgsQABiKBRixAxiDAcICBhAAGBYYHsICCBAAGIoFGIYDwgIIEAAYFhgeGA_CAgUQIRigAcICBxAhGKABGArCAgUQIRifBeIDBBgBIEGIBgGQBgE&sclient=gws-wiz-serp
Should we bet the planet on this bit?
brush
(57,495 posts)They're still being found today. Might be hydrogen deposits too. Let's reserve denials while exploration is being done.
I doubt the discovery on the link is the only one on the planet.
NNadir
(34,662 posts)You would think that if there were oodles of hydrogen, we would have come across it before now.
Even if it existed on any meaningful scale - to the extent that it exists, it is almost certainly generated by the geological steam reformation of fossil fuels - it would fail the sustainability test.
We've just passed through 50 years of wishful thinking, hype, fantasy, and handwaving.
For the week beginning 11/05/23 the concentration of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere was 419.28 ppm. In June of 1983, the month that the International Journal of Hydrogen Energy began publishing, a little over 40 years ago, the concentration in the week beginning June 12, 1983, that same concentration was 345.72 ppm. That's a difference of almost 74 ppm in just 40 years.
How long are we supposed to wait around to find out if there's so much geological hydrogen that it will matter?
You know what, the planet burst into flames while we all waited for magic to happen. It isn't going to happen. We need to get serious and not grasp insipidly at straws.
I have no use for more wishful thinking.
brush
(57,495 posts)There's no guessing on that. I think I'll stay open minded and see it the scientists and geologists will develop ways to do the same on hydrogen deposits.
NNadir
(34,662 posts)With people dying in the streets from extreme heat, with glaciers on which billions of people depend for the water supply disappearing, the seas rising, vast ecosystems dying, ancient forests bursting into flame, I can't think of anything more horrible than "waiting to find out."
JFC.
brush
(57,495 posts)One would think you'd be in favor of that. What's with all the negativity.
NNadir
(34,662 posts)I would suggest opening a good geology book.
brush
(57,495 posts)as stated by this from your previous post:
It's commendable all right but all of that you mention is caused by human activity, namely by burning oil and gas for fuel for over a century now, yet you say waiting to see if scientists/geologists can find clean hydrogen energy sources is delusional.
How silly.
IMO searching for any energy sources that don't cause people dying in the streets from extreme heat, with glaciers on which billions of people depend for the water supply disappearing, the seas rising, vast ecosystems dying, ancient forests bursting into flame is commendable, not delusional.
NNadir
(34,662 posts)...with references to papers in the primary scientific literature, which I browse in most of my free time. There are several scientific journals, including the one cited in the OP, for which I scan the table of contents in its entirety, making notes of papers of interest, either professionally or connected with my personal interest in the science connected with environmental issues and clean energy engineering.
I hear all sorts of wild schemes to which I am asked to respond. I was reading issues of the International Journal of Hydrogen Energy decades ago in connection with thermochemical production of hydrogen.
It's your option to cheer for the chimera du jour. We certainly have lots of hydrogen nonsense around here and all over the planet, despite the fact that the physical properties of hydrogen are absymal leading to considerable safety risks and it's not primary energy.
From my perspective, all the "green" or "white" hydrogen hooey is a bait and switch effort to increase reliance on dangerous fossil fuels and to degrade the environment further and faster.
Believe what you want, but I'm of the opinion, that we were out of time 20 years ago and thus are out of time now.
brush
(57,495 posts)only water vapor and warm air as exhaust and is considered a zero-emission vehicle.
If the hydrogen used comes from pure hydrogen wells instead of hydrogen produced by a process that burns more energy to produce it than the energy it delivers, it of course makes sense to call it hooey.
But I still say it's silly to dismiss the recent discoveries of green hydrogen deposits in Africa and Australia (I posted a link to view that earlier). They could be all over the planet just as oil deposits are.
As for being out of time now as you say, I guess we should all just give up, throw up our hands and wait for the planet to become uninhabitable?
Even though I don't agree with you on the hydrogen fuel issue, I didn't expect you to be a "Chicken Little" type.
hunter
(38,931 posts)OTTAWA, Nov 9 (Reuters) - Montreal-based renewable energy firm TES Canada H2 Inc will build a C$4-billion ($2.9 billion) green hydrogen project in Quebec that is expected to create 200 permanent jobs and reduce 3% of the province's carbon emissions by 2030, a source familiar with the project told Reuters on Thursday.
TES Canada, a unit of Tree Energy Solutions, is expected to make an announcement on the project on Friday with Canadian Industry Minister Francois-Philippe Champagne, the source said, declining to be named because details are not yet public.
The green hydrogen project will use a wind and solar farm to produce most of the energy it needs, and it will create over 1,000 temporary jobs during the construction period, in addition to permanent positions, the source said.
--more--
https://www.reuters.com/sustainability/climate-energy/canada-firm-build-c4-bln-green-hydrogen-project-quebec-source-2023-11-09/
Direct link to TES Canada H2 Inc: https://tes-h2.com/
They are going to take "recycled" carbon dioxide, add hydrogen made by electrolysis using solar and wind power, and turn it into synthetic natural gas and other fuels.
The world is saved!
It's a greenwashing machine, of course, with a thermodynamic profile similar to this: