Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Science
Related: About this forumMore Christmas Chemistry: Key Odorants of Frankincense, cis and trans Olibanic Acids
Earlier today I posted on the chemistry of Myrrh: Christmas Chemistry: Synthesis of an Essential Oil from Myrrh
To repeat what I said in that post:
It's probably not entirely Kosher for an atheist to comment on Christian Scriptures, but as a cultural affectation, based on my Christian upbringing and my long participation in American consumer culture, I will anyway.
That post was based on the recent ACS "Molecule of the Week;" this one is based on the previous week's "Molecule of the Week."
Olibanic acids
Excerpts:
Frankincense, also called olibanum, is a resin obtained from trees in the genus Boswellia that grow in Africa and Asia. One of the first aromatic materials used by humans, it dates to the late fourth millennium BCE. It and myrrh1 are familiar as gifts offered to the Christ child by the Magi in the Christian gospel.
Surprisingly, the main odor ingredients in frankincense were only recently discovered. In the 2016 seminal article on this discovery, Nicolas Baldovini and collaborators at the University of Nice Sophia Antipolis (France), Albert Vieille SA (Vallauris, France), and the University of Turin (Italy) reported the isolation and characterization of ( + )-cis- and ( + )-trans-2-octylcyclopropanecarboxylic acids, which they called olibanic acids after frankincense’s alternative name. The 3-D image above represents the more fragrant cis isomer.
The olibanic acids constitute only ?0.2 wt% of the oil derived from frankincense; but the authors’ olfactory tests showed that they are responsible for the aroma of the resin, which they describe as the “smell of old churches”. The researchers synthesized the two (+)-diastereomers as well as their (–)-counterparts, which do not exist in frankincense. The synthetic (+)-diastereomers are identical with the natural compounds that were extracted from B. carterii and B. frereana, species that grow in mountainous regions on both sides of the Gulf of Aden and the Red Sea...
Surprisingly, the main odor ingredients in frankincense were only recently discovered. In the 2016 seminal article on this discovery, Nicolas Baldovini and collaborators at the University of Nice Sophia Antipolis (France), Albert Vieille SA (Vallauris, France), and the University of Turin (Italy) reported the isolation and characterization of ( + )-cis- and ( + )-trans-2-octylcyclopropanecarboxylic acids, which they called olibanic acids after frankincense’s alternative name. The 3-D image above represents the more fragrant cis isomer.
The olibanic acids constitute only ?0.2 wt% of the oil derived from frankincense; but the authors’ olfactory tests showed that they are responsible for the aroma of the resin, which they describe as the “smell of old churches”. The researchers synthesized the two (+)-diastereomers as well as their (–)-counterparts, which do not exist in frankincense. The synthetic (+)-diastereomers are identical with the natural compounds that were extracted from B. carterii and B. frereana, species that grow in mountainous regions on both sides of the Gulf of Aden and the Red Sea...
The structure of these stereoisomeric acids is somewhat unusual for a natural product: They have highly strained cyclopropyl rings.
The chemistry, structure and synthesis of these molecules is described here:
C. Cerutti-Delasalle, M. Mehiri, C. Cagliero, P. Rubiolo, C. Bicchi, U. J. Meierhenrich, N. Baldovini,, The (+)-cis- and (+)-trans-Olibanic Acids: Key Odorants of Frankincense Angew. Chem. Int. Ed. 2016, 55, 13719.
A scheme for synthesis is shown in that paper.
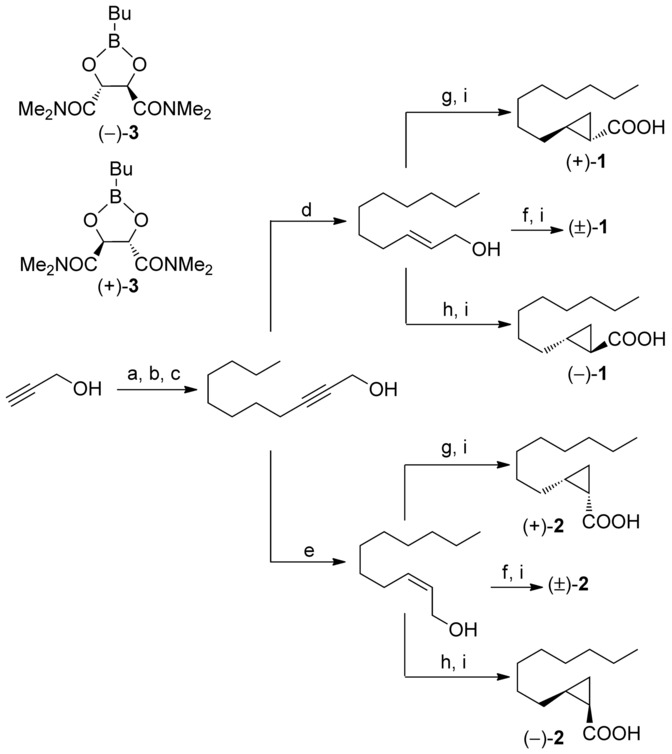
The four diastereomers are on the right.
The caption:
Scheme 1
Reagents and conditions: a) 3,4-dihydropyrane, Amberlyst® 15, Petroleum ether, RT, 7 h, 89?%; b) NaH, DMSO (4 equiv), THF, RT, 15 h, then 1-bromooctane, RT, 29 h, 82?%; c) Amberlyst® 15, MeOH, 45?°C, 40 h, 84?%; d) LiAlH4, THF, reflux 2 h, 64?%; e) H2, P2-Ni, 1,2-ethylenediamine, MeOH, RT, 17 h, 87?%; f) Et2Zn, CH2I2, n-hexane, ?35?°C?RT, 13 h; g) ? )-3, CH2Cl2, ?15?°C, then Zn(CH2I)2-DME, CH2Cl2, ?15?°C?RT, 15 h; h)
? )-3, CH2Cl2, ?15?°C, then Zn(CH2I)2-DME, CH2Cl2, ?15?°C?RT, 15 h; h)  + )-3, CH2Cl2, ?15?°C, then Zn(CH2I)2-DME, CH2Cl2, ?15?°C?RT, 15 h; i) Jones reagent, acetone, RT, 20 h (yields over two steps: ( ± )-1, 56?%; ( + )-1, 67?%; ( ? )-1, 79?%; ( ± )-2, 68?%; ( + )-2, 66?%; ( ? )-2, 80?%). DME=dimethoxyethane, DMSO=dimethylsulfoxide, THF=tetrahydrofuran.
+ )-3, CH2Cl2, ?15?°C, then Zn(CH2I)2-DME, CH2Cl2, ?15?°C?RT, 15 h; i) Jones reagent, acetone, RT, 20 h (yields over two steps: ( ± )-1, 56?%; ( + )-1, 67?%; ( ? )-1, 79?%; ( ± )-2, 68?%; ( + )-2, 66?%; ( ? )-2, 80?%). DME=dimethoxyethane, DMSO=dimethylsulfoxide, THF=tetrahydrofuran.
Reagents and conditions: a) 3,4-dihydropyrane, Amberlyst® 15, Petroleum ether, RT, 7 h, 89?%; b) NaH, DMSO (4 equiv), THF, RT, 15 h, then 1-bromooctane, RT, 29 h, 82?%; c) Amberlyst® 15, MeOH, 45?°C, 40 h, 84?%; d) LiAlH4, THF, reflux 2 h, 64?%; e) H2, P2-Ni, 1,2-ethylenediamine, MeOH, RT, 17 h, 87?%; f) Et2Zn, CH2I2, n-hexane, ?35?°C?RT, 13 h; g)
Note that the ? symbol may appear until DU4 allows for the import of symbolic structures. EarlG and Elad have it on their agenda for sometime in the future, whereupon the symbols will be restored to the text.
Some text from the paper:
The olfactory evaluations showed that ( + )-1 and ( + )-2 were both extremely potent odorants, and their GC-O analysis enabled us to confirm unambiguously that they were the main contributors of the characteristic old churchlike, olibanum endnote odor zone in the olfactogram of the frankincense sample. The relative detection threshold of all four isomers was determined by GC-O with a panel of four judges. The data of each individual panelist showed the same tendency: their qualitative olfactory properties were similar but ( + )-2 was the most potent odorant, followed by ( ? )-2 and ( + )-1, and the weakest was ( ? )-1...
To the best of our knowledge, natural 2-alkylcyclopropane-1-carboxylic acids are scarce: trans- and cis-2-pentylcyclopropane-1-carboxylic acids (4 and 5) are trace constituents of patchouli EO9 and (1S, 2R)-5 has been detected in Mentha gracilis EO (Figure 3)...
...It is surprising that 1 and 2 remained unidentified up to now, while a detailed survey of the litterature shows that many phytochemical studies focused extensively on the volatile fraction of frankincense.14 When considering the main species used in incense burners (i.e. Boswellia carterii, B. sacra, B. papyrifera, and B. frereana) the major volatiles are generally either classical monoterpenes (?-pinene, ?-thujene, limonene) or octan-1-ol and its acetate.14 Interestingly, some old references noticed that both of these types share some common olfactory properties,4b thus suggesting that they may contain common odorants...
To the best of our knowledge, natural 2-alkylcyclopropane-1-carboxylic acids are scarce: trans- and cis-2-pentylcyclopropane-1-carboxylic acids (4 and 5) are trace constituents of patchouli EO9 and (1S, 2R)-5 has been detected in Mentha gracilis EO (Figure 3)...
...It is surprising that 1 and 2 remained unidentified up to now, while a detailed survey of the litterature shows that many phytochemical studies focused extensively on the volatile fraction of frankincense.14 When considering the main species used in incense burners (i.e. Boswellia carterii, B. sacra, B. papyrifera, and B. frereana) the major volatiles are generally either classical monoterpenes (?-pinene, ?-thujene, limonene) or octan-1-ol and its acetate.14 Interestingly, some old references noticed that both of these types share some common olfactory properties,4b thus suggesting that they may contain common odorants...
Again, I wish you the happiest of holiday seasons consistent with your cultural preferences near or at the winter solstice, and the happiest New Year which will include the reelection of Joe Biden to be President of the United States.