Science
Related: About this forumA Device About Which I Wish I Knew When I Was a Kid, the Taylor Vortex Apparatus.
Looking back at my overly long life, I will briefly comment on this paper: Comprehensive Study on Particle–Wall Interactions: From Microscopic Parameters to Macroscopic Implications Yueming Wang, Yuxing Wang, Shuo Zhang, Minmin Zhou, and Lunbo Duan
Industrial & Engineering Chemistry Research 2023 62 (50), 21740-21749.
This is probably of very esoteric interest, but is of personal interest to me because I recall a very happy evening in the lab after months of working to try to crystallize a very difficult compound in a long series of trial and error cosolvent systems, cooled and heated at various rates, finally, on a cold night in a poorly heated facility in California - it was cold enough in the lab to require layered sweaters - I turned by back on a stirring flask full of a solution of the freshly synthesized compound in question under scrupulously dried nitrogen and when I turned around, the flask was filled with highly pure crystals. They were like rocks, hard, beautifully white, and on analysis, incredibly pure. (These compounds could only be purified by crystallization, since the goal was to produce industrial scale amounts of the product; they were very sensitive to moisture and in fact, decomposed in the presence of water.)
That was a good night.
I wish I could tell you that this surprising success owed to theoretical insight, but it was just a lot of slogging trial and error.
Papers about crystallization - in particular the theory of crystallization as well as the theory of devices to manage it always catch my eye, decades after I had to do it myself.
We had nothing sophisticated in terms of crystallization devices in our lab, and in fact, I wouldn't have even thought of getting any such device since I was blissfully unaware that they existed. I was, after all, just a kid.
This paper thus caught my eye.
From the introduction:
ARTICLE SECTIONSJump To
Peptides represent a unique class of pharmaceutical compounds intermediate in molecular weight between small molecules and proteins, with desirable biological activities, including antioxidant, antihypertensive, antithrombotic, antilipogenic, antibacterial, and anti-inflammatory effects. Special properties, including low toxicity and high specificity, make these molecules of particular interest to the food and pharmaceutical industries. (1?3) At present, more than 80 polypeptide drugs such as oxytocin, vasopressin, and cyclosporine are approved in the global market, with more in preclinical and clinical development. (3) With the fast development in upstream processes such as the synthesis of these peptides, fermentation, and cell culture technologies, the production bottlenecks shift to downstream purification and formulation steps. The current mainstream purification method is chromatography, (4) but it has some significant shortcomings, such as low throughput, large water consumption, and high cost. The current purification cost can account for more than half of the total production cost in the biomanufacturing process. In addition, chromatography is difficult for scaling up in the manufacturing of peptides. (5,6)
Crystallization is a widely used separation and purification technology in the pharmaceutical industry, which presents higher titer and better scale-up capability than traditional packed-bed chromatography. (7,8) The application of crystallization as an alternative pathway for the separation and purification of peptide drugs has become attractive for industrial manufacturing. In addition, from the perspective of drug formulation, crystalline products have the advantages of higher purity, physicochemical stability, and dissolution characteristics compared to standard protein formulations such as aqueous solutions and amorphous precipitated lyophilizes. (9) However, it is challenging to form a highly ordered crystal structure with large, complex, and flexible peptides, proteins, and even monoclonal antibodies. (10) The nucleation and growth kinetics are relatively slow. (11) Yang et al. (12) proposed a set of optimized crystallization conditions that still need 24 h to produce monoclonal antibodies CD20 in needle-shaped crystals in a very reproducible manner with high yield. Therefore, how to obtain high yield and high-quality crystalline products in a short residence time becomes a crucial question. Recently, the research on peptide crystallization has focused on crystallography and structural determination to develop an efficient technology for the separation and purification of biomacromolecule crystals from biological mixture materials. (13) In addition, from the perspective of industrial crystallization, the stability, crystallinity, size, particle size distribution, crystal shape, and purity of the crystalline products are also important. Downstream processing of poor crystalline products such as tableting, filtration, and washing are often difficult, resulting in the lack of process robustness and poor final product quality. (14) Therefore, it is extremely important to control and strengthen this crystallization process, especially for the nucleation and growth process. (15)
The Taylor vortex device, which can generate a constant shear environment, has been widely used in aviation, water treatment, pharmaceutical engineering, and chemical industry. (16) The Taylor vortex is a fluid motion existing in the annular gap of two coaxially positioned cylinders, and through the relative rotation of the inner and outer cylinders, a series of secondary vortices that are alternating in the axial forward and negative, axisymmetric, and orderly arrangement superimposed on the shear flow are generated in the annular gap, as shown in Figure 1a. (17) In the Taylor vortex device, the mixing strength is controlled mainly by adjusting the speed and gap width. The Taylor vortex in the crystallizer can improve the mixing behavior and the flow field distribution, which can effectively enhance heat and mass transfer. (18) Moreover, the Taylor vortex can produce a uniform shear force, which can effectively strengthen the crystallization process and obtain crystalline products with a uniform particle size distribution. (19) It is reported that the Taylor vortex has been applied to a variety of processes in industrial crystallization such as reaction crystallization, drowning-out crystallization, and cooling crystallization. (19) Kim et al. (20) demonstrated from both experimental and modeling prospects that Taylor vortex has a better mass transfer effect than random turbulence, thus promoting stable polycrystalline nucleation and phase transition of histidine. Wang et al. (18) used the Taylor vortex to improve the mixing behavior, so that the velocity distribution of the flow field was uniform, and hydroxyapatite with uniform particle size distribution was obtained efficiently, which provided a reference for the synthesis of inorganic micronanomaterials. The Taylor vortex has also been reported to have great advantages in enhancing the crystallization of biomacromolecule drugs. Sun et al. (21) obtained high-quality lysozyme and thaumatin crystals by using Taylor vortex. Tao et al. (19) successfully solved the crystal structure of lysozyme with 1.86 Å diffraction accuracy by combining Taylor vortex with poly(ionic liquids) and obtained 86% yield within 3 h, which greatly improved the production efficiency of lysozyme crystals.
Vancomycin (glycopeptide drug vancomycin hydrochloride) is widely used in the treatment of infections caused by methicillin-resistant Staphylococcus aureus and penicillin-resistant pneumococcus, (22) which is known as the ?last line of defense? of antibiotics. (23) The chemical structure of vancomycin is shown in Figure 1b. At present, the industrial purification and separation of vancomycin are mainly realized using chromatography technology. (1) There is limited research on the industrial crystallization of vancomycin. In the studies by Kim et al., (24) a combination of cooling, salting-out, and antisolvent crystallization took 24 h to reach 95% yield. Pu et al. (25) investigated the behavior of vancomycin salting-out crystallization and determined the conditions for the formation of octahedral massive crystals in 96-well plates, further transferring the results to batch crystallization (1.5 and 15 mL) in a sealed vibrating vessel. However, incubating at room temperature for 24 and 48 h under gentle shaking, only 50% yield could be obtained. Therefore, it is important to explore alternative strategies to amplify and accelerate the crystallization process of vancomycin octahedral crystals to increase the yield and productivity.
In this study, vancomycin was selected as the model peptide to perform salt-out crystallization in static conditions, the mixing-tank (MT) crystallizer, and the Couette–Taylor (CT) crystallizer under equal crystallization conditions. The influences of thermodynamic (concentration and temperature) and kinetic (shear rate and gap size) parameters on the crystalline products (crystal shape, yield, and crystal size distribution) in the CT crystallizer were investigated.
Chromatography on an industrial scale is rather expensive, and more importantly, very dirty since it involves huge quantities of often nasty solvents in flow systems as well as finicky and easy to destroy solid phases in columns.
Here is a picture of the Taylor device and the structure of vancomycin:
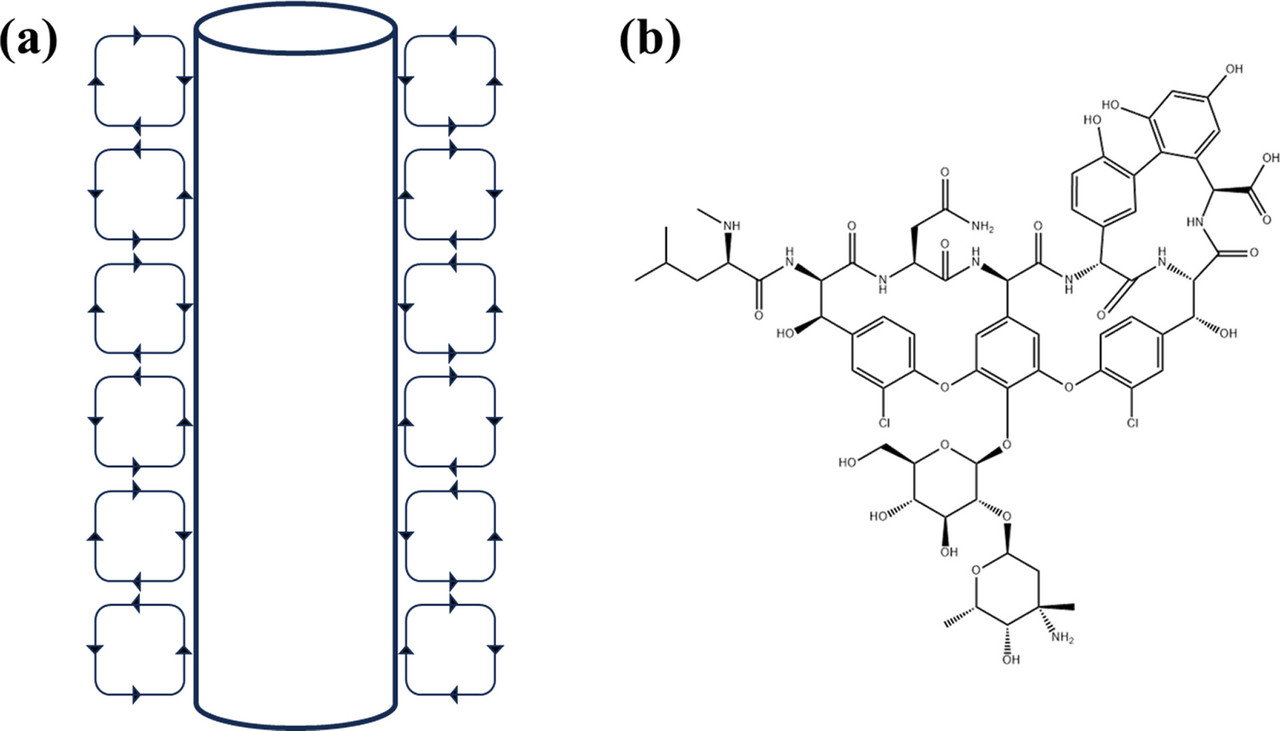
The caption:
A comment on the structure of the "peptide" vancomycin, a very beautiful molecule. Calling it a "peptide" is somewhat disingenuous, since, although there are lots of peptide bonds (amides) only two of the amino acid functionalities are coded amino acids, a leucine and an asparagine. Clearly there are moieties biosynthetically arising from tyrosine, and in one case a tyrosine-like phenylglycine, but frankly the phenolic ether bonds are more reminiscent of lignin structures as opposed to peptide structures. In particular, even without special knowledge, I'm quite sure this molecule could never be synthesized by straight up peptide synthesis. It is almost certainly made by fermentation procedures using cultured organisms.
Schematic cartoons of the apparatus:
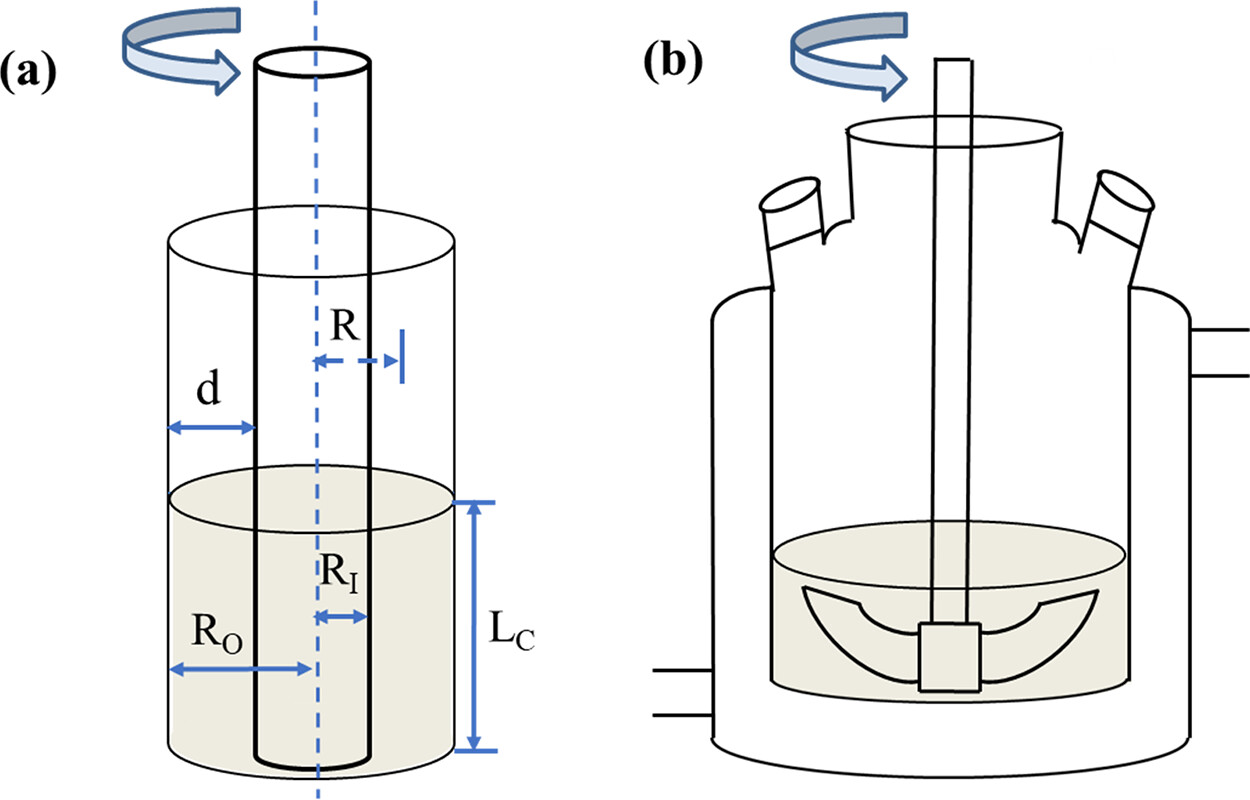
The caption:
Were I aware of this technique, I might well have been able to jury rig a mixing tank crystallizer; in the event what I was doing was not entirely dissimilar, but I had no idea about the theory.
The paper, by contrast, has a fairly nice description of theory. My approach to crystallization was entirely empirical.
Of course, if I'd had some sophistication back then, I might not have taken the pleasure in succeeding that I ultimately enjoyed.
The Taylor vortex is the result of study by the physicist G.I. Taylor, whose discovery late in life of the Taylor cone was a critical understanding leading to the development of modern ESI mass spectrometry.
I believe I wrote about about G.I. Taylor some place in this space, but can't locate the post. The point was that at an advanced age people can still do great things, for example a certain President of the United States who happens to occupy the office now and who succeeded another President of the United States who was incompetent through his entire life and now is truly even more senile.