Science
Related: About this forumHas a UC Berkeley chemistry lab discovered the holy grail of plastic recycling?
Could this be a first step in mitigating the damage plastics do to our environment?
Ultimately: A plastic that's recylable?
Story by Susanne Rust • 4d • 5 min read
snip
But new research from a team of chemists at UC Berkeley suggests a glimmer of hope when it comes to the thorny problem of recycling plastics — one that may allow us to have our cake, and potentially take a very small bite, too.
The group has devised a catalytic recycling process that breaks apart the chains of some of the more commonly used plastics — polyethylene and polypropylene — in such a way that the building blocks of those plastics can be used again. In some cases, with more than 90% efficiency.
The catalysts required for the reaction — sodium or tungsten — are readily available and inexpensive, they say, and early tests show the process is likely scalable at industrial levels. It uses no water and has fewer energy requirements than other recycling methods — and is even more efficient than manufacturing new, or so-called virgin, plastics, the researchers say.
Link to story.
Here's another story from The Independent:
snip
The trillion dollar question: Have scientists found the holy grail of plastic?
Analysis: Scientists from Berkeley Lab claim to have made a plastic that can be recycled endlessly
To recycle or not to recycle? That is the difficult question many of us face several times a day when deciding which bin to put our empty packaging in.
Even when we do recycle, our waste still often ends up in the wrong place.
The most recyclable plastic, PET (polyethylene terephthalate), is recycled as little as 20 per cent of the time.
The rest of it ends up being incinerated, or put in landfill or the ocean. Marine plastic costs the world almost £2 trillion a year in damaged and lost resources, according to research last month.
snip
Link to abstract at Science:
Polyolefin waste to light olefins with ethylene and base-metal heterogeneous catalysts
Wicked Blue
(6,655 posts)in their home.
Just feed in whatever plastic you want recycled. Out come blocks of plastic that can be used for 3D printing or whatever.
Not likely, I realize that. But it's nice to dream.
ColinC
(10,683 posts)But I don’t know that something like that isn’t way out of the question 🤔
Wicked Blue
(6,655 posts)Some are recyclable with current technology. Others are not. Unless science comes up with some new way of re-using plastic of all types, a household would need a device for each kind of plastic.
![]()
SCantiGOP
(14,239 posts)There’s a story about a big breakthrough in plastic recycling.
Maybe this one will work, and I would prefer that technology solve it, but support government doing it if necessary.
NJCher
(37,883 posts)I was hoping to read more stories about breakthroughs, based on your comment that there is a breakthrough once a year, but I could only find about 1 breakthrough story for every two years.
There needs to be a breakthrough once a month, considering the magnitude of this problem.
prefer that technology solve it, but support government doing it if necessary.
who mostly funds these studies? I thought the government funded them but I didn't get a definitive answer from a relatively brief search. I see the U.S. Dept. of Energy invested $13.4 million two years ago.
LauraInLA
(1,306 posts)and other stuff that’s difficult to recycle. They work with a lot of different partners to recycle these things. I love them! You can check if they’re in your area at Ridwell.com. There’s also Terracycle.com, which accepts all kinds of things like razor blades/disposable razors, many companies’ plastic containers, and other things. As much as I’ve tried to stop buying plastic, it seems there’s always more.
Just checked--Ridwell is not yet here, but I put my name on the list to get information on when they do move into NJ/NY area.
Terracycle: wow! I am looking forward to studying that site.
I serve as an adviser for a number of public school environmental clubs and we take on a project every year. These sites look like they could offer some ideas the students might like.
LauraInLA
(1,306 posts)NNadir
(34,664 posts)...waste.
A picture from the text:
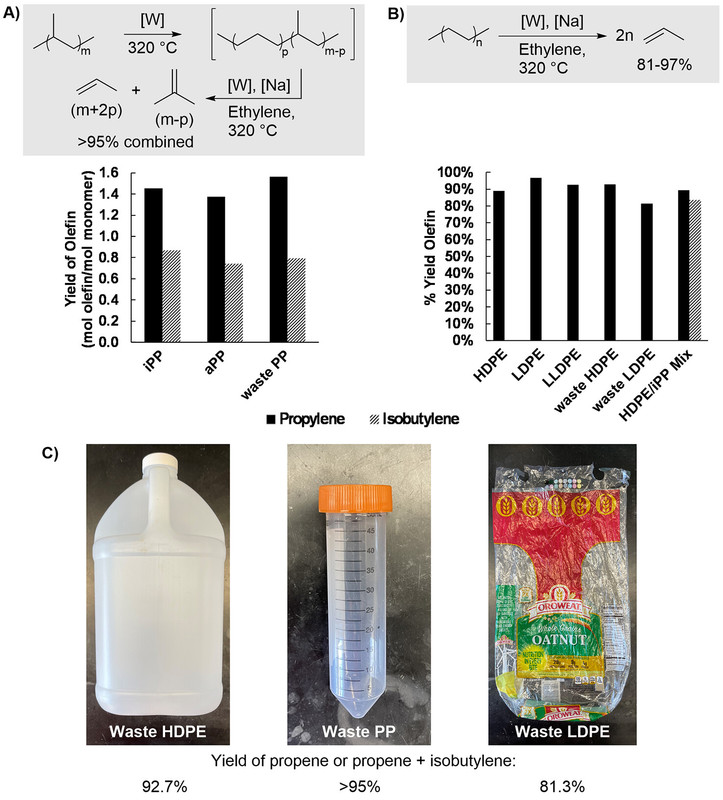
The caption:
(A) Yields of CIE on various examples of commercial and waste polypropylene: PP (1.00 g, 23.8 mmol of monomer units), WO3/SiO2 (400.0 mg, 0.1075 mmol W), and Na/?-Al2O3 (400.0 mg, 1.740 mmol Na) heated to 320°C under 15.0 bar of ethylene (175 mmol) for 90 min. (B) Yields of CIE on various examples of commercial and waste polyethylene: PE (1.00 g, 35.7 mmol of monomer units), WO3/SiO2 (400.0 mg, 0.1075 mmol W), and Na/?-Al2O3 (400.0 mg, 1.740 mmol Na) heated to 320°C under 15.0 bar of ethylene (175 mmol) for 90 min. (C) Photographs of sources of waste subjected to CIE: waste HDPE (left), waste PP (middle), waste LDPE from a bread bag (right).
(CIE = catalytic cracking and isomerizing ethenolysis)
To my mind, separations are still required; relying on consumers to understand the chemical nature of polymers clearly isn't working.
While I credit the paper as being innovative, and perhaps useful in some settings, to my mind the most likely to succeed approach to this very serious problem would be high temperature (supercritical or at the edge of supercriticality) steam reforming, which oxidizes everything to CO + H2, "syn gas," hydrogenation of the syn gas to methanol, followed, as necessary by demand, the MTO (methanol to Olefins) reaction. This would only be sustainable in the case nuclear energy were the source of the supercritical water, or sub-supercritical steam.
As to whether I believe a "holy grail" has been obtained, my answer is a firm "no."
NJCher
(37,883 posts)I looked for a less sensational headline but it wasn't available. I thought about editing it myself--should have done that.
OTOH, they didn't say it was the holy grail. They posed it as a question. Actually, rather clever on their part.