Science
Related: About this forumA Very Interesting New Technique for the Analysis of Alloys.
I came across a very novel analytical technique in a paper I was reading today from my general reading, this one: Chemical Fingerprinting of PM2.5 via Sequential Speciation Analysis Using Electrochemical Mass Spectrometry Lili Song, Luyao Zhong, Ting Li, Yufei Chen, Xinglei Zhang, Konstantin Chingin, Ni Zhang, Hui Li, Liyun Hu, Dongfa Guo, Huanwen Chen, Rui Su, and Jiaquan Xu Environmental Science & Technology 2024 58 (43), 19362-19371
I live day to day pretty much in the world of mass spectrometry, not so much in the environmental space, but in biological matrices connected with drug development, and I never heard of this technique and so I went immediately to the references.
This paper seems to be the paper in which the technique was first introduced: Sequential Formation of Analyte Ions Originated from Bulk Alloys for Ambient Mass Spectrometry Analysis Jiaquan Xu, Tenggao Zhu, Konstantin Chingin, Yuhui Liu, Hua Zhang, and Huanwen Chen Analytical Chemistry 2018 90 (23), 13832-13836.
The introductory text:
To date, alloys are normally dissolved using various chemical reagents to form analyte solutions prior to elemental analysis. The sample solutions are usually offline analyzed by either atomic emission spectrometry (AES), (15) atomic absorption spectrometry (AAS), (16) or inductively coupled plasma mass spectrometry (ICPMS). (17) The offline procedures render a significantly long time for sample manipulation and pretreatment, (18) resulting in a low analytical throughput. Laser ablation-inductively coupled plasma mass spectrometry (LA-ICPMS) or laser ablation electrospray ionization mass spectrometry has been attempted for online alloy analysis (19) but require expensive high-energy laser generator and hard-to-reach standards for quantitative analysis. No matter if the alloy is online or offline treated, ICPMS provides only elemental information due to the highly energetic ionization process occurring inside the ICP torch. (17) Ideally, soft ionization is preferable for mass spectrometry analysis of alloys, particularly in the cases where the organics on the alloy surface are of analytical interest. (20)
Herein, electrochemical ionization mass spectrometry (ECI-MS) was developed to directly characterize alloys at the molecular level without tedious sample pretreatment, for which sequential formation of metal ions of bulk alloys via electrolysis was a crucial step. As schematically shown in Figure 1, charged electrolytic solution (e.g., H2O/CH3CN, 1:1, v/v) was injected at 2.0 ?L/min by a syringe pump to flow through a 50 ?L fluid tight microelectrolytic cell (MEC), inside which the organic chemical species on the alloy surface took precedence over the rest of the material to be extracted into the solution. Once the metal was exposed to the solution, a potential (e.g., 1.0 V) was applied to the working electrode inside the MEC. Consequentially, the elemental components were successively converted into the corresponding metal ions by tuning the electrolysis potentials, ensuring the high selectivity and specificity for metal ion generation. (21) Note that the electrolysis potential was floated on the ESI voltage (±3.0 kV), such that the ESI high voltage brought no interference to the electrolysis. (22,23) Once the metal ions were formed, they reacted immediately with specific organic ligands added in excess into the electrolyte solvent to form metal–organic complexes, which were carried by the solvent flow toward the outlet of the MEC to produce ambient ions, with the assistance of the pneumatic system and the electric field, for mass analysis using a LTQ-Orbitrap-MS instrument...
I was speaking with a friend today about the difference between graduate school in Pakistan (where he went to school) and in the US, in particular about the "hands on" part. Apparently in Pakistan, at least according to what he told me, analytical work is performed for graduate students by hired technicians who run the instrument and supply the data, whereas in the US, many times one has to jury rig and experience "hands on" approaches. Of course, in the early days of science, this sort of thing was required for all research. (One did not search the internet for a supplier of instrument x or instrument y, material z, sample m, and one did not do on line tutorials.)
Just this morning, before going to work I was reading how, during the Manhattan project, Lise Meitner's nephew (and co-discover or nuclear fission with her) Otto Frisch, was working with a criticality determination device known as a Godiva, when he realized that the carbon and hydrogen in his body was reflecting neutrons when he hovered above the device, causing the mass with which he was working to go critical. Happily he noticed the detectors lighting up and pushed the enriched uranium blocks apart before he got a fatal dose of radiation. He was apparently unhurt, although later people would be killed in criticality experiments, most famously Louis Slotin. (My son's Halloween costume for his nuclear engineering Halloween party, apparently was to go as Slotin, with a fake demon core that lit up with a red LED) These people, the real people working on the Manhattan Project, were working with instruments they built, including the Godiva device. (The Godiva device, which is still in use today for criticality experiments because it was "naked" in the sense that it lacked a neutron moderator, something Frisch's body inadvertently became.)
The authors of the paper under discussion are Chinese, and it's pleasing and fun to see that they built their device, albeit using some commercially available parts, to interface with a commercial mass spec instrument.
From the paper:
The analysis device consisted of a MEC (about 50 ?L), a nebulization system, and a mass spectrometer. The MEC employed two platinum wires (diameter = 500 ?m) as the working electrode and auxiliary electrode and Ag/AgCl as the reference electrode, respectively. In order to ensure the reproducibility of experimental behavior and to avoid the need for the cleaning of the electrode surface, a new disposable MEC unit was assembled and used in every experiment. Metal was connected to the anode or acted as a bipolar electrode placed between two platinum wires. (24) Electrolyte was injected into the MEC by a pump. During the detection of metal materials, a potential (E) floated on +3 kV high voltage was applied to the working electrode by CHI 660D electrochemical workstation. When potential E is high enough to meet the requirement of metal electrolysis, metal would transform into metallic ions. Then, the electrolyte contained metal ions was sprayed into the MS by pneumatic nebulization for analysis. The distance between the spray emitter and the entrance of the MS is 1.0 cm.
A schematic of the device:
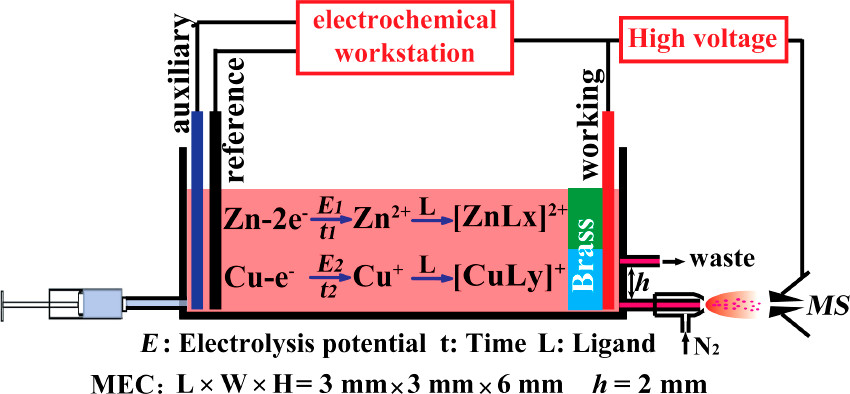
The caption:
OK, they didn't build everything from scratch, but the put things together in a new way, and this is beautiful science, because it's creative, different, and in its own way, represents a kind of courage.
We will need courage in the scourging our nation is about to experience.
The paper just made me feel better, and while this is esoteric, well, again, reading it, just made me feel better. There will be life beyond; I may not live to see it, but there will be life beyond the horrible times coming.
Have a nice evening.
WestMichRad
(1,805 posts)as to whether they addressed the capability of the ECI-MS methodology in determining non-metallic components of alloys. Specifically, non-conductive components such as some polymers. Does the MEC technique have the ability to liberate these components from the alloy? (Hopefully that’s not a stupid question!)
In any case, sounds like a very clever and promising technique.
NNadir
(34,654 posts)...in to it."
This technology is, first and foremost involved with metals. People do not generally appreciate this, but electrochemistry can be, and sometimes is, a method of separations, since one can adjust voltages to affect the redox (reduction/oxidation) potential which is generally varies from metal to metal and indeed, from oxidation state to oxidation state for multivalent metals like manganese which features a large number of oxidation states, ranging from +7 (in permanganate) to a formal -3 state in a complex ions known.
Your question inspired me to consider these, and I came across an interesting paper which refers to something (obliquely) about I haven't thought about in some time, zintl salts, which struck me as interesting some time ago with thermochemical cycles in nuclear plants to split water. (It reminds me that I should write that thought down so my son has it when I die.)
Ellis, Adventures with Substances Containing Metals in Negative Oxidation States Inorg. Chem. 2006, 45, 8, 3167–3186.
But let's turn to your question, on reflection, with a little digging around, I can imagine that this approach involving organic polymers could be of interest, although to my knowledge, while depolymerization via electrochemistry has been explored, it has not been, again to my knowledge, to ESI mass spec.
There is a whole field of electrochemical organic chemistry.
I can think of a project (which actually didn't go well) on which I worked a few years ago, where it might have been of interest to have at least thought about it, although probably it would have been a bit too "out there."
Much of what has been written about electrochemical depolymerization concerns the second most common polymer on Earth, after cellulose, which is lignin, the polymer that gives wood much of its strength but is also present in plant matter that is not wood.
Here is an open sourced paper on depolymerization of anthropogenic polymers: Electrochemical C?H/C?C Bond Oxygenation: A Potential Technology for Plastic Depolymerization In theory, I suppose that one could couple this process to mass spec to monitor it, although I'm not sure that it has been done.
Thank you again for your question. It made me think.